BET - Measuring Areas Using Molecules
Created | Updated Nov 4, 2002
BET comes from the initials Brunauer, Emmett and Teller1, the men who invented a straight-forward method, and a complicated-looking accompanying theory, to determine the effective surface of solid materials with complicated shapes, such as porous powders, by using adsorbed gas molecules as rulers. The observation of the so-called adsorption and desorption isotherms is used to determine the amount of gas molecules adsorbed to a surface. Knowing the size of a molecule, one can then calculate the entire effective surface. In scientific literature one will commonly bump into the terms 'BET-isotherm' or 'BET-method' when this approach has been used.
OK, But... Why?
Why would one need a new way of measuring areas, if one can do that using primitive trigonometry and a pocket calculator? Simple areas, like the area of a tennis-court or the surface of a doughnut, are indeed relatively easy to determine using plain geometry (cf Calculating the Volume and Surface Area of Various Solid Objects). More complicated surfaces, however, like the entire surface of a powder, or the entire surface of a grinding-stone, that is, the surface around every little bump it is made of, are a lot more difficult, if not impossible, to measure or calculate using geometry.
An Analogy - The Area of a Tennis Court
Before delving into questionably important scientific abracadabra, it is worthwhile taking a look at the following analogy. It is intended for those readers that are not so familiar with scientific formalism. It will demonstrate how to determine the surface area of a tennis court covered with clay.
One could imagine a tennis court covered with a thin film of glue. Now, after unloading a truck of clay onto that surface and shaking it a bit, the surface will be covered with clay corns. The excess of clay, namely the corns that are not glued to the surface, can be easily removed. The next step is to dissolve the glue, and pour the remaining clay, that is, the clay that was once glued to the surface, into a recipient. From the weight of one corn one can deduce how many corns are in the recipient altogether by weighing it. One should now carefully measure the width of one individual corn of clay, and calculate its effective area: that is, the area it will cover on the surface. Multiplying that number with the number of clay corns will yield the surface of the tennis court. If the tennis court is not perfect, that is, if it has tiny bumps, eg, along the demarcation lines, the surface will be slightly larger than the area calculated using traditional geometry. Replacing 'corns of clay' for 'gas molecules' and 'tennis-court surface' by 'solid material surface' will result in the mechanism used in the BET-Method.
The BET-Method in Principle
The problem Brunauer, Emmett and Teller were facing in the early 1930s was not so connected to surfaces of tennis-courts but to the surfaces of more complicated-looking solids. Like: how big is the surface of a powder - and what if each corn of that powder has an indeterminate number of holes and pores and cracks? The method they developed is, in principle, quite straight-forward. Namely to use the surface of gas molecules as a ruler, as described in the analogy above.
Normal Behaviour
One of the basic properties of gas molecules is that they like to stick to surfaces2, all one has to do is to find out how many gas molecules are stuck to the surface of the solid material in question. From the effective area of each molecule one can then obtain the whole area with good accuracy. To determine that amount of molecules, one can look at their adsorption/desorption isotherms. Isotherms are volume and pressure relations at a constant temperature. According to the gas state equations, pressure (p) is (in a first approximation) directly proportional to the number (n) of molecules a gas is made of:
V·p = RT·n
In this relation, V is the volume where the gas is in - it stays constant if one uses a closed rigid recipient, like a pot of marmalade. R is the gas-constant, which is nothing but a conversion factor, so that the units match. T is the absolute temperature (in K), which in the case of isotherms is also a constant. Hence, if one pumps more and more gas molecules into the pot the pressure will rise linearly. Conversely, if molecules are pumped out of the pot the pressure will decrease linearly. So far, this all sounds incredibly reasonable.
Adsorption and Desorption
Experiments showed that when pumping molecules out of a recipient, all goes linearly well and as expected normal until a certain level of 'emptiness' is reached. After that point the curve starts to deviate from the 'normal' behaviour. The pressure does not decrease at the same pace as before. Instead, it decreases a lot slower. The same happens in the other way round, when an empty pot is slowly filled with some gas molecules: in the beginning the pressure seems to increase only very slowly. After a certain point it increases a lot faster, and the pressure then shows again its 'normal' linear behaviour.
The explanation for this deviation is the following: a gas molecule can only contribute to increase the pressure if it is diffusing around freely. If it sticks to some surface it will not be 'available' to increase the pressure. And that is exactly what happens. The very first molecules that are pumped into the recipient will stick to its surface. One observes a phenomenon called adsorption. All molecules adsorb or stick to surfaces, the question is only how strongly they will do that. After the whole surface is covered with one layer of molecules a second layer will build up. This layer, however, will not be bound as strongly to the first one, because the nature of the interaction is more similar to the one present in the gas - that is, the new molecules will not be interacting with the surface, but with other similar molecules, since the surface is one layer away. In principle this layering might go on forever: a third layer would build up, and a fourth one and so on. Normally after the first two layers the interactions responsible for the adsorption become so small that any new atom added will more likely contribute to increase the pressure than sticking to some layer. At that point the increase in pressure starts to become 'normal'.
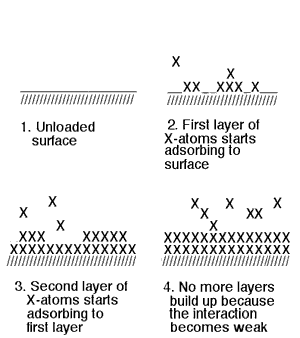
In the schematic representation above this process is illustrated. In the first frame a schematical zoomed-in view of the fresh unloaded surface is depicted. In the second frame the molecules (marked by an X) start adsorbing to the surface. Note that of the eight added molecules only two will contribute to an increase of pressure. In the third frame the first layer is complete and a second layer starts to build up upon the first one. In this example 24 molecules have been added to the recipient and only four contribute to the pressure. In the fourth frame both layers are complete. If one assumes that the formation of a third layer of molecules is improbable, then any new added molecule will contribute to an increase of pressure. That's the point where the behaviour becomes normal: one molecule, one addition to the pressure.
Using this behaviour, one can determine the point where the curve starts deviating from the normal behaviour. When starting with an unloaded surface, the first nick of the curve will indicate the point where the first layer has just finished building-up. A second nick would indicate the building-up of a second layer, and so forth. When going the other way round, ie, unloading the surface, the last nick will indicate the point where the first layer just starts unloading. Normally it is easier to go from the unloaded surface to the loaded surface, because it's easier to identify the first nick than identifying the last since one will never be sure if the observed nick is actually the last.
Details, Types of Isotherms, Hysteresis and Surface Textures
As easy as it might seem, it is the deviation from the normal adsorption behaviour that will allow the observer to draw conclusions on the texture or porosity of the surface. And this is also the point where the theory starts to become complicated. This will not be discussed at full detail in this Entry.
In general there are six types of adsorption/desorption isotherms3. The conventional adsorption/desorption isotherm plot shows the volume of adsorbed molecules against pressure, instead of plain volume against pressure. The reason for this is that the point where the layers build up is visualised better, and because this form is apparently easier to connect to the BET-equation. Normally a scientist doing BET-measurements will not calculate that curve using complicated equations over and over again. Instead he or she will check the pattern of the curves with standard pictures.
Since there is no way to add graphics to h2g2 in an easy way, some imagination will be required to follow the next description of the curves. All six curve types start from zero and rise steeply. All of them bend to the 'right' at some point, become shallower and have a characteristic plateau, which is the point where no more molecules can be adsorbed by the material. Four of them have an additional first plateau right at the beginning, which is where the first layer builds up. The remaining two are very atypical and only restricted to some special exceptional cases. To measure the surface area of the material in question all one has to do is to identify the first nick in the curve and take note of the volume, which is the volume of gas molecules used to form the first layer.
Sometimes one might observe hysteresis. Hysteresis occurs when loading a surface is easier than unloading it (for example if there is a pore or a tight crack), the curve for the loading will be steeper than the curve for the unloading. This makes things a bit more complicated, and that's where details start to become important. These details allow conclusions on the texture of the surface - for example about the porosity of the surface, and even about the shape of the pores. Further interpretations can be made but are usually difficult.
There are commercially available BET-measuring devices. In principle they are made of a sample chamber, where one can put the powder to be measured, and which is connected to a gas inlet, a vacuum pump and a (electronic) barometer. The first step is to evacuate the chamber, so that all surfaces get cleaned from adsorbed molecules. Then a small volume of gas is added in a controlled manner, normally automatically, and the pressure measured. This step is repeated several times. For desorption measurements gas is pumped out and the pressure measured. It is important to note that every time gas is added or removed from the chamber one will have to wait some time until equilibrium is reached, only then the measured pressure yields a reliable value.
There are more advanced models for adsorption and desorption isotherms, however, they didn't find as broad application as the BET method. BET is employed as a standard measurement for technical powders, as used in catalysis for example. Normally BET-measurements are carried out with different gas molecules (CO2, N2, and Ar). The values can differ slightly for each gas employed, since their geometry and adsorption characteristics vary. For thit reason the gas used is often given in parenthesis along with the area measured (and other features like the porosity).
Vm/(p-p0)·1/V = 1/c[1/p+(c-1)/cp0]
Here, V is the total volume of molecules added, Vm is the volume of gas molecules corresponding to the monolayer, p the pressure and p0 the saturation vapour pressure, c is a constant related to the adsorption heat and it has something to do with probability of adsorbing or desorbing. This equation is indeed a bit complicated and was just included for the sake of completeness.

